This is not meant to replicate exact mathematical values - impossible withot a lot more work and area! - it is simply to test if thermally conductive but thermally opaque plates cause anomalous temperature rise on the heater.
It should be noted that if the ambient were at 200C instead of 20C then there would still be 1.88 watts heating it up from the ambient. 1.88 watts produces a temperature increase of 50C above AMBIENT
Basically:
a nuclear core generates 235W/sqm will emit 235 W/sqm to space
Surround this with a steel shell
When the system has reached stability the shell (which is the same surface area (approx) as the core emits the generated 235 watts (if it did not then the system would not be at stability).
However the shell must emit the same quantity of radiation from both sides. the inward flux is the same as the outward at 235 W/sqm. The core must therefore heat up in order that the shell now receives 235+235 W/sqm
I.e. the core is emitting 470W/sq m
See:
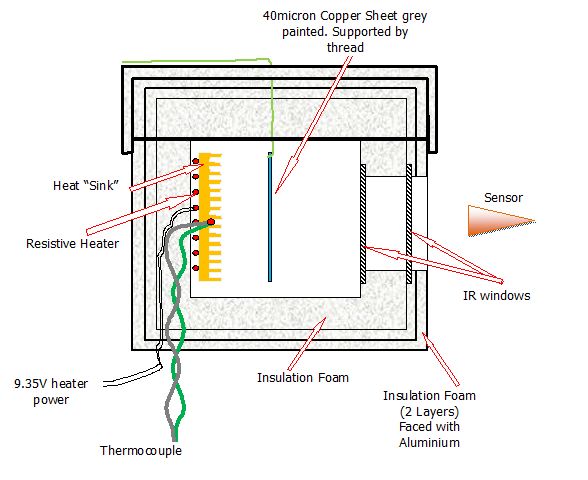
http://wattsupwiththat.com/2013/02/06/the-r-w-wood-experiment/ and http://wattsupwiththat.com/2009/11/17/the-steel-greenhouse/ This test simplifies the greenhouse to a heater and copper plate the same size as the heater. All measurements are made using a digital thermometer with a resolution of 0.1degC The heat box is similar to that used before - just 2 layers of aluminium foil backed thermal insulation added to outside of box. A removable thin copper sheet, painted grey, is either hung in front of the heater or removed from the box. The sensor is placed at exactly 15mm from front of insulation round box. The temperatures of the hot plate and the sensor are unfortunately affect fractionally by room temperature - This is visible in the results below
Heating began from room temperature 21C. It reaches a stable temperature of 73.6degC with the copper sheet present and 72.2 degC with the plate absent.
The sensor temperature at the point the copper sheet was removed was 21.6degC the final temperature measured by the sensor was 22.2degC The emissions from the hot box is therefore no less with the copper removed compared to with the copper in place despite the change in hot plate temperature. The new setup - This allows for 2 copper plates to be hung between the heater and the fron IR double glazed window One of the plates can be replaced with a single plastic IR transmissive sheet. Note All hanging sheets are approximately the same size as the heater. The heater is dissipating 1.88 Watts Box Dimensions
137x130x80 mm - external
80x80x40 mm - internal
heated plate and hanging sheet dimensions
35x35x6 mm approx
The results - Room temperature controlled to +-1deg C
Here we see that with 2 copper plates the temperature is 75.7degC
The sensor in front of the IR window measures 23.25degC
With 1 copper plate the temperature is 75.1degC
The sensor in front of the IR window measures 23.6degC
With 1 copper plate the temperature is 74.05degC
The sensor in front of the IR window measures 24.6degC
With 1 IR transparent plastic plate the temperature is 74.5degC
The sensor in front of the IR window measures 23.6degC
The most significant results here are the 1 copper plate vs the 1 IR transmissive plastic plate.
The disturbance caused by inserting a plate is the same in both cases
but the heater runs 0.6degC hotter
It is interesting that 2 plates allow the heater to reach a higher temperature than just one (as the iron gh predicts)
Unless the iron greenhouse is accepted I do not see how this result could be explained.
Errors -
GHGs are present
The heater is loosing heat to the back wall
There is not a vacuum between heater and plate.
The box still looses too much heat through its sides.
Ambient has too much effect.
lgl says: March 23, 2013 at 4:42 pm
thefordprefect why didn’t you use bigger plates, all the way to the walls so that hot air couldn’t leak from the warm side to the cold side? ------------- TFP: this would have changed the radiating area, I felt it best to keep the plate close to the heater and for the area radiating to be constant so the ir window restrictive size would have similar effect. --------------------------------------- A C Osborn says: March 23, 2013 at 4:16 pm Sorry, your results appear to completely disprove the iron ball/shell theory unless you can show by calculation that 50% of the radiation from the Heat Source equals a rise in the temperature of the heat source of only approximately 1.25 degC. Do you really think that the 1.25 degC increase in the heat source represents the 50% increase in the iron ball temperature from the Willis theory? ----------------------------- TFP: This sort of real world kitchen table top experiment in no way can EXACTLY replicate the iron greenhouse thought experiment. As I said in the write up, perhaps the most important thing is the the 2 single plate runs the internal stucture of the warm box is the same (a restrictive plate changes the convection in the box in a similar way, so what explains the 0,6degC rise in temperature when the copper plate is present? I was not looking for exact energy flows (for example I knew that the IR windows are not 100% transmissive, I knew the box is loosing heat through its walls, I do not know what the thermal capacity of the heater is, etc. All this is experiment does (and was expected to do) is show a warming where there shousd according to the slayers be none ------------------ A C Osborn says: March 23, 2013 at 5:01 pm The temperature rose because the interior conditions of the box have been changed. For instance the GHGs (air) between the heat source and the plate could have been heated more due to Reflected radiation from the plate, which is not a perfect black body and not re-emitted radiation, which in turn would heat up the source slightly. --------------------------------- TFP yes the internal conditions have change but that is why I tried a IR "invisible" plate in place of the copper. The IR loss in this plate did cause a slight warming of the heater but no where near as much as the single copper plate. but also remember that the plate will be cooler than the heater and slayer theory says energy cannot travel from cooler to hotter! ------------------------------- tallbloke says: March 23, 2013 at 5:23 pm Right up until the last line I was with you. ------------------------ TFP mmmmm! I suppose you are right I'll modify that! removed!! I believe that this shows that willis's iron greenhouse model is likely to be valid. Some bolometer stuff http://home.strw.leidenuniv.nl/~kenworthy/teaching/dol2011/10_DOL_Bolometers.pdf http://home.strw.leidenuniv.nl/~kenworthy/teaching/dol2011/11_DOL_Bolometers_part_2.pdf Some radiative transfer stuff: http://www2.ups.edu/faculty/jcevans/Pictet%27s%20experiment.pdf A good description at the end! The final proof? Some really silly stuff: http://climateofsophistry.com/2013/03/08/the-fraud-of-the-aghe-part-11-quantum-mechanics-the-sheer-stupidity-of-ghe-science-on-wuwt/ |

























Wilis, Joe Public has it exactly right. If you want to know what happens to the flux from a cooler source when it hits a warmer source, the answer is exactly nothing. It is not absorbed, but immediately re-emitted, transferring NO heat.
All these analogies are amusing but ignore Second Law.